"Safety is difficult to know,”
By Tyler Durden: In the interest of "transparency", Pfizer CEO Dr. Albert Bourlas released a statement on Friday outlining the timeline for when its experimental COVID-19 vaccine might be ready for approval for regular, non-emergency use. Much to Trump's chagrin, CEO Dr. Albert Bourla said the earlier the company expects to apply for an emergency-use approval from the FDA would be the third week of November, after the election has come and gone.
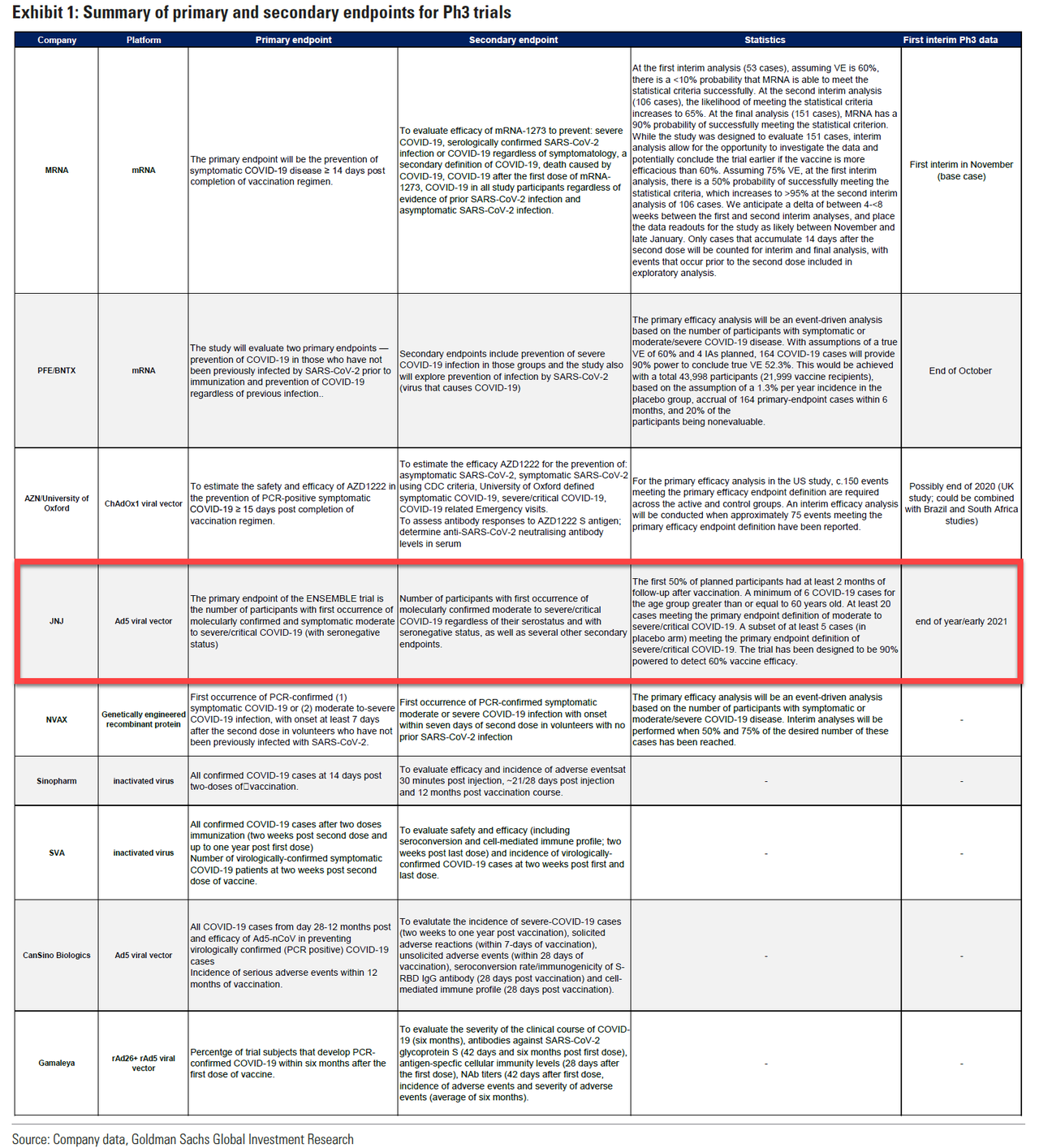
The news capped off what was a busy week for vaccine news, which started Monday night with reports that Phase 3 trials for Johnson & Johnson's COVID-19 vaccine have been paused after a participant came down with an "unspecified" illness.
While JNJ executives insisted their vaccine candidate likely wasn't the cause, they brought in investigators and are now working to get the trial going again as quickly as possible.
If what happened with the AstraZeneca-Oxford vaccine is any guide, it could be weeks before US regulators sign off on allowing the trial to resume. AstraZeneca-Oxford trial in the US has been paused for more than a month.
Back in September, AstraZeneca briefly halted global studies after UK
regulators investigated a pair of patients who came down with symptoms
of a rare illness which scientists worried might be connected with the
vaccine. Trials in the UK and elsewhere restarted days later, but in the
US, regulators have refused to allow the trials to resume.
Already, the time lost by JNJ and AstraZeneca-Oxford has pushed them out of the lead in the race to apply for an emergency use authorization from the FDA. Vaccine candidates being developed by Moderna and Pfizer-BioNTech have taken the lead.
But as Bloomberg pointed out in a piece published Saturday morning, both the AZ and JNJ vaccines relied on the same technique: the so-called adenovirus vector which was also used by the Gameleya Institute's vaccine (aka Sputnik 5) and at least one of the leading Chinese vaccines.
Still, if these investigations determine a link between the illnesses and the vaccines, this could bolster public skepticism, something that surveys have shown is already alarmingly high.
And this year, with Covid-19 vaccines entering strongly into the politics of the hour, transparency and trust are key to fighting a virus that’s hit more than 39 million people globally and hamstrung economies. If concerns about side effects in experimental vaccines in trials using adenoviruses are validated, it could boost skepticism in the general public and raise questions for other drugmakers.
It would also amount to a considerable setback to adenovirus vector vaccines.
As Bloomberg explains, the adenovirus vaccine vector is nothing new to medicine. They are well studied and versatile, according to Bloomberg. Humans have been shown to easily tolerate them, which is what initially attracted scientists to experiment with this method for COVID-19. A J&J vaccine based partially on the method was recently approved to guard against Ebola.
However, the technique already has a couple of conspicuous blemishes on its safety record. Here's one example: An AIDS vaccine based on the technique was abandoned after the virus was potentially tied to increased infections among those who received it.
In other experiments, though, there were disappointing results. In 2008, a vaccine using an adenovirus developed by Merck & Co. to prevent HIV was tied to increased infections among some who received it in a trial. Merck abandoned the shot, and several similar programs fell by the wayside.
US regulators are still investigating illnesses related to 2 trial participants in the UK, who showed symptoms of transverse myelitis, a rare condition causing inflammation of the spinal cord.
If investigators in the current trials determine the cause of the episodes is related to the vaccines, they would look for potential links to the adenovirus approach as well as to the spike protein the vaccine is designed to make to prepare the immune system for a real infection, according to Michael Kinch, a vaccine specialist at Washington University in St. Louis. At this point, he said, there’s not enough information to know.
The drug companies insist that the illnesses are unrelated, and trials of the AstraZenaca-Oxford vaccine resumed weeks ago in the UK, Brazil and South Africa. These Phase 3 trials have recruited tens of thousands of participants; many of them are the size of small towns.
"Is this just random chance?" Kinch said. "First and foremost, there’s bad luck. If it turns out there’s a correlation and a causation, then the conversation very quickly turns." J&J said it’s still learning about the illness of the participant in its trial. The adenovirus in its experimental Covid shot has been used worldwide in more than 110,000 people, according to Paul Stoffels, the company’s chief scientific officer. “We are building very quickly on a very large safety database of the carrier,” Stoffels said in an interview before the trial was paused.
Bloomberg goes into the history of the adenovirus vector, which stretches back to 1953, and includes at least one tragic death of a trial subject who had a severe immune reaction to an early trial on gene therapy techniques.
When it comes to the most cutting-edge technologies, safety can be difficult to gauge, one expert acknowledges. Although according to scientists who are experts in the field, modern techniques involve much smaller doses of the adenovirus, which makes safety "not an issue," according to one.
Still, with such new technologies, "safety is difficult to know,” said Baden, who’s worked in the HIV vaccine field for decades. “If you’ve studied it in 1,000 people, you don’t know a 1-in-10,000 risk; if you’ve studied it in 10,000 people you don’t know a 1 in 100,000, and so on.”
Still, if US investigators determine that the illnesses exhibited by the patients in the J&J trial along with the AZ-Oxford trial were caused by the vaccines, it could potentially set gene therapy efforts back by years.
While also creating serious credibility issues for vaccines in the US that could endure beyond the COVID-19 crisis.

No comments:
Post a Comment