In this two-part paper, we aim to give an overview on COVID-19 related abnormal blood clots, how they form, how to detect them early, and how they're being treated
By Dr. Yuhong Dong & Dr. Jordan Vaughn: Since mid-2021, unusual, lengthy blood clots found in the vessels of COVID-19 patients and jab recipients have been reported across the world.
“We as embalmers are seeing some strange clots since the COVID outbreak. These clots are very rubbery feeling and very long as they exit the veins that we use during the embalming procedure. They really appear to be like earthworms. I have never seen this in my career until now,” Larry Mills, a licensed embalmer and funeral director in the State of Alabama, told The Epoch Times.
Other embalmers confirmed similar findings and spoke on the condition of anonymity.
Richard Hirschman, Alabama funeral director and embalmer since 2001, was one of the first to bring attention to this phenomenon. He said that prior to COVID perhaps 5 to 10 percent of people had these clots. Now more than half of the bodies he sees have them.
One embalmer, licensed since 2001, said in an interview, “I can tell you with certainty that the clots Richard has shown online are a phenomenon that I have not witnessed until probably the middle of last year. That is pretty much all I have to say about it. I have no knowledge as to what is causing the clots, but they did seemingly start showing up around the middle of 2021.”
Where do these strange, fibrous clots come from? How do they form?
A Condition With Over 200 Symptoms
Doctors have realized, since the early days of the pandemic, that COVID-19 is not just a lung disease, but also an endothelial and vascular disease.
Physicians have summarized a list of unusual clinical observations of COVID-19 including but not limited to severely hypoxic (low oxygen) patients despite relatively normal lung compliance upon examination, thrombotic complications, and consistent autopsy findings of blood clots (thrombi) in the microcirculation of the lung.
After an acute COVID-19 infection, over 200 different lingering symptoms have been reported for long COVID, which can persist for about 6–24 months.
This is perhaps the largest number of symptoms reported with a medical condition so far.
The most frequent symptoms include breathlessness, fatigue, brain fog, cognitive dysfunction, muscle aches and pains (myalgia), sleeping difficulties, and anxiety or depression.
The chronic, relapsing nature of long COVID is mainly caused by immune dysregulation, hyperinflammation, oxidative stress, and mitochondrial dysfunction.
But how could it be, and why? Clues have emerged since 2020.
Blood Clots Causing Symptoms
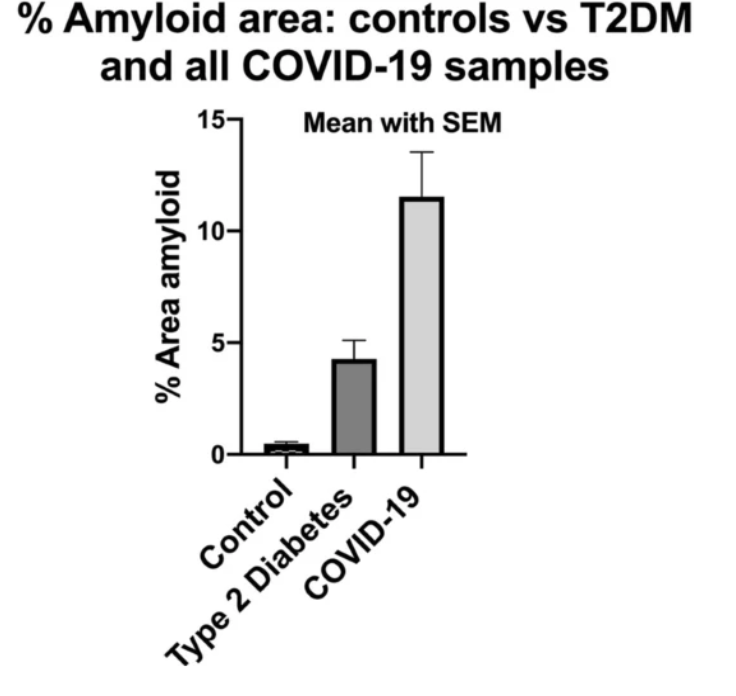
In November 2020, a report with findings of increased microclots in COVID-19 patients versus healthy or diabetic patients could reasonably explain the breathlessness, fatigue, and post-exertional malaise syndrome.
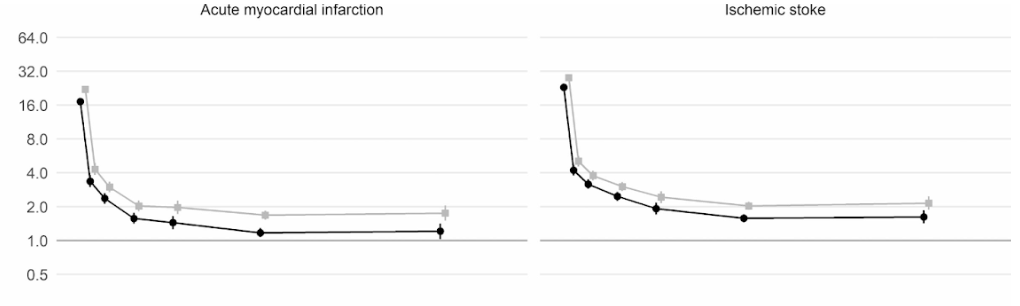
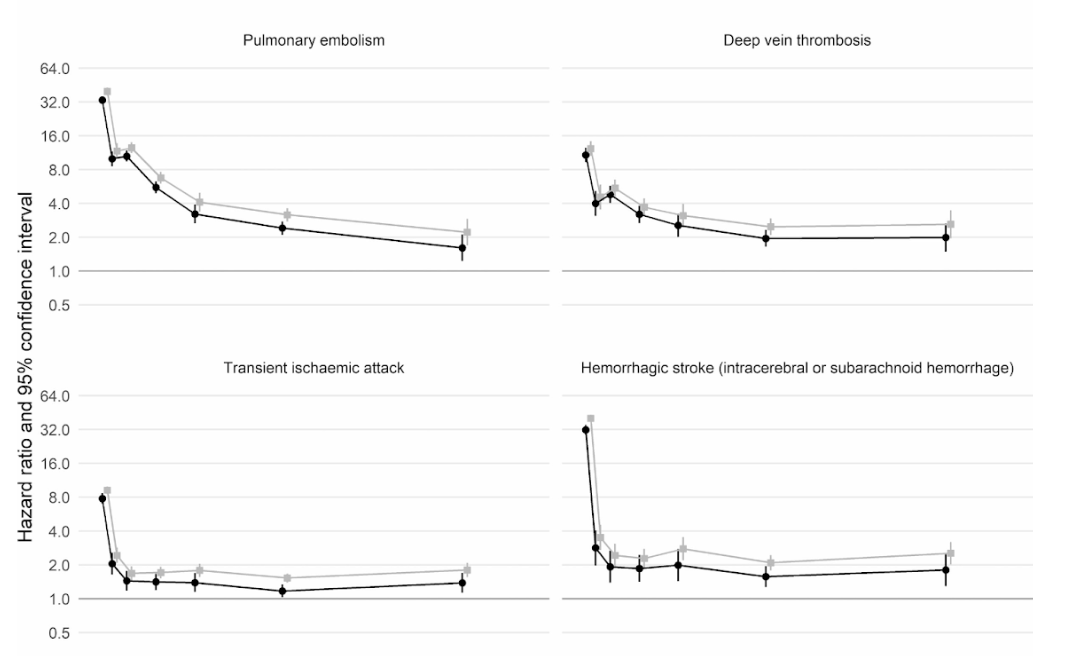
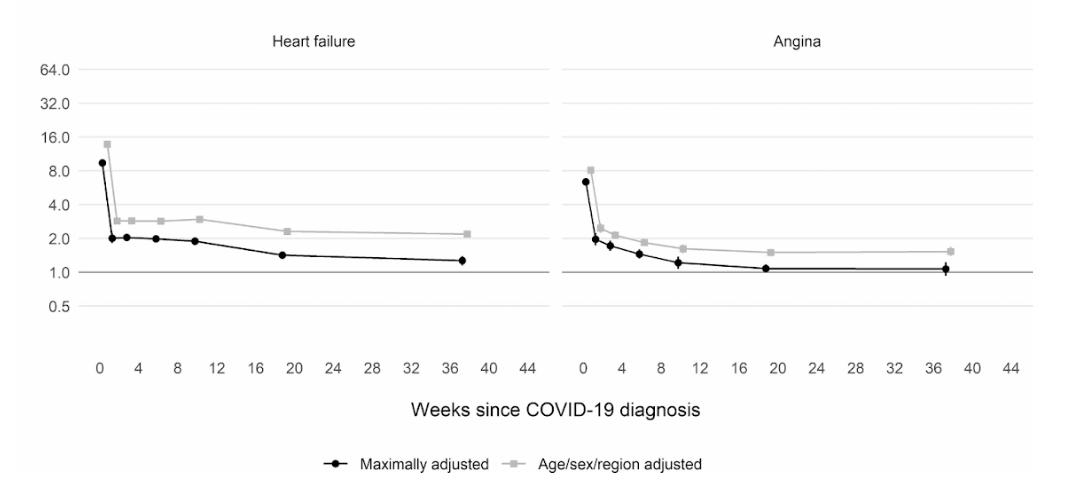
Moreover, a large scale cohort UK study based on 48 million adults in England and Wales found that in the first week after a COVID-19 diagnosis, the risk of an arterial blood clot was nearly 22 times higher than in someone without COVID-19, and 33 times higher for a venous clot condition.
An artery clot is the kind that could cause a heart attack or ischemic stroke by blocking blood flow to the heart or brain.
This has led to an estimated 10,500 additional cases of clot-related problems, i.e. about 7,200 additional heart attacks or strokes, and 3,500 additional cases of pulmonary embolism, deep vein thrombosis, or other venous problems.
Even though that risk drops sharply to less than four times higher than someone without COVID in the second week, it remains high (2x) even up to 49 weeks after the initial diagnosis. This is especially so in regards to the risk of deep vein thrombosis. These are clots that form in large veins.
Spike Protein: The First Domino Toppled
Blood is a liquid that circulates under pressure through the blood vessels in our whole body, like the water flowing through the house that you then use to shower, do the dishes, and so on.
Following a vascular injury, any blood “leaking out” must rapidly be converted into a gel (a “clot”) to fill in the hole and minimize further blood loss.
Normally, the plasma portion of blood contains a collection of soluble proteins that act together in a series of enzyme activation events that result in the formation of a fibrin clot. This process is protective, as it prevents excessive blood loss following injury.
Unfortunately, the blood clotting mechanism can also lead to unwanted blood clots inside blood vessels (pathologic thrombosis), e.g. heart attack or stroke, both of which are a leading cause of disability and death in the world.
COVID-19’s way of causing abnormal blood clots has spurred many discussions since early 2020.
It appears that the virus’s unique spike protein triggers the cascade via many “non-traditional” pathways.
The spike protein’s direct invasion of the epithelium cells is the first domino toppled.
Subsequent cascade effects finally cause the blood clotting.
Spike Protein Impairs Epithelium Cells
SARS-CoV-2 enters our cells via a protein receptor called the angiotensin-converting enzyme 2 (ACE2).
Endothelial cells (ECs), express an abundance of ACE2. ECs reside on the inner surface of every blood vessel across our entire body, making them a direct target of the virus infection.
Studies showed that spike protein itself can damage the structure and function of ECs, including impairing the mitochondria and downregulating the protective molecule ACE2 on ECs.
Researchers observed that both the S1 and S2 parts of the spike protein can induce human ECs to express a peak of pro-inflammatory cytokines (IL6, IL1B, TNF-alpha, and chemokines CXCL1, and CXCL2).
Subsequently, the release of cytokines initiates the switch-like molecule (E-selectin) on the endothelial cell membrane, allowing them to attach with immune cells, thereby initiating subsequent disease processes.
Additional studies on the spike protein showed that it activates the EC inflammation dependent on integrin ⍺5β1 and NF-κB signaling pathways and subsequent expression of leukocyte adhesion molecules.
Cytokines are small proteins secreted by cells (mainly T cells and macrophages). They have many specific names, and serve as a communicator between cells (cell signaling) for further action. Cytokines are the “mailmen” of the body that connect and communicate between cells.
The spike protein can induce a breakdown of connecting proteins between endothelial cells of blood vessels, which disrupts the integrity and function of our blood vessels.
Brain ECs also strongly express ACE2. Spike protein has similar toxic effects on brain ECs, inducing neurological symptoms (brain fog, cognitive decline).
Spike Proteins Trigger the Clotting Cascade
Many other cells, including lung epithelial cells, enterocytes lining the small intestines, and cardiac pericytes, all express ACE2.
Spike proteins don’t only activate epithelial cells (EC) and promote localized inflammation. They also promote systemic inflammation as ACE2 is almost everywhere inside our major organs and tissues.
Consequently, more pro-inflammatory genes are expressed. More and more immune cells are attracted and sent to the injured or infected tissues (vessels in the lung, heart, gut, etc).
A number of subsequent events collectively contribute to the clotting cascade:
- Complement-mediated inflammation of epitheliums (endothelialitis): Spike proteins docking on ACE2 ECs activates the complement pathway and coagulation cascade, resulting in a systemic endothelialitis (lung injury) and procoagulant state (tendency to develop blood clots).
- As the complement destroys the endothelium, the procoagulant von Willebrand factor (vWF) and FVIII are released. A significant increase of vWF can form multimers that promote thrombus growth. vWF is secreted mainly from endothelial cells and from a-granules of platelets (megakaryocytes derived). This is comparable to the string in the “beads and string” of a necklace where the beads represent platelets.
- Platelet storm: Platelets are a small fragment of the
megakaryocytes. The complement anaphylatoxins C3a and C5a activate
platelets and increase the production of tissue factor further promoting
a clotting forming state.
ACE2 receptors are present on platelets, and this may contribute to the massive platelet aggregation, which is a characteristic of severe COVID-19 infection. - Activation of neutrophils leads to formation of neutrophil extracellular traps (NETs), a process sometimes referred to as NETosis, which contributes to thrombosis.
- EC injury is compounded by toll-like receptor (TLR) activation by viral RNA recognition, with resulting increased reactive oxidative species (ROS) production. The increased ROS further upregulates the expression of vWF.
- Spike protein can induce expression and secretion of a series of clotting proteins which cascades into the clotting process, including factor (F)-V, thrombin, and fibrinogen to promote clotting process.
Spike Protein Dysregulates RAAS, Worsening the Clotting State
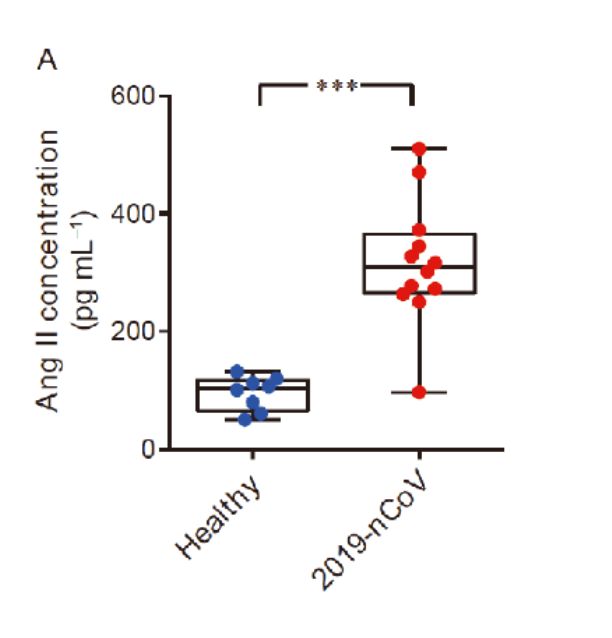
Due to the spike protein directly interacting with ACE2 expression, COVID-19 patients showed an elevated level of serum angiotensin II indicating a dysregulation of the RAA system (renin angiotensin aldosterone system, or RAAS).
Traditionally, people think angiotensin II is a neurohormone that stimulates the constriction of vascular smooth muscle cells and is responsible for salt and water balance.
However, there have been abundant studies supporting the idea that angiotensin II is capable of initiating and upregulating inflammatory responses, worsening the clotting state.
In a regulated and self-limited immune response, these mechanisms help to calm down the local injury, with subsequent healing and returning to a resting EC state.
However, for predisposed COVID-19 patients or vaccinated people, the factors strengthening clot formation are way heavier than healing mechanisms, all of which lead to an escalating thrombotic cascade.
Here is a short summary of the first scene of the clot story: spike induced endothelial disruption, massive amounts of vWF released, a subsequent platelet storm, hypoxia induced upregulation and activation of vWF, fibrous network from neutrophil extracellular traps (NETs), as well as increased angiotensin II level, all adding up to initiate thrombogenesis.
This is how the clotting mechanism comes to be. Furthermore, the upcoming second scene takes another pivotal part in the whole story.
A COVID vaccine instructs the cells to produce large quantities of spike proteins. Normal biochemical and physiological processes are “hijacked” in order to make an abnormal amount of these spike proteins.
These abnormal amounts of spike protein have more surprising direct effects on clots.
Spike Proteins Directly Disrupt the Clot Dissolving Mechanism
In a normal healthy person, the body will, in the presence of a blood clot, break it down by a process of fibrinolysis. This is a natural healing and balancing mechanism to prevent an abundance of blood clots.
During this process, Tissue Plasminogen activator (TPA, coming from the endothelium) helps plasminogen change into plasmin and then generate d-dimer (a small protein fragment left when a blood clot dissolves).
It has been discovered that fibrinogen in blood can clot into an abnormal “amyloid” form of fibrin that (like other β-rich amyloids and prions) is relatively resistant to proteolysis (fibrinolysis).
This is strongly manifested in the platelet-poor plasma (PPP) of individuals with long COVID. The extensive fibrin amyloid microclots can persist.
In an outstanding study by Grobbelaar published in Bioscience Reports in August 2021, the biomarker S1 (or the intruding part of the spike protein) alone can induce fibrin resistance to fibrinolysis, leading to unopposed microclot formation.
When spike protein S1 was added to healthy PPP, it resulted in structural changes to β and γ fibrin(ogen), complement 3, and prothrombin. These proteins were substantially resistant to trypsinization in the presence of spike protein S1.
Hence, the results suggest that the presence of spike protein in circulation may contribute to the hyper clotting status, and may cause substantial impairment of the clot dissolving process.
Such lytic impairment may result in the persistent large microclots that people have reported and which have been found in plasma samples of COVID-19 patients.
These microclots block up capillaries, and thus to limit the passage of red blood cells, and hence oxygen exchange, which can actually underpin the majority of these symptoms.
Spike Proteins Form Amyloid-Like Substance
Furthermore, to everyone’s surprise again, spike proteins are identified to present seven amyloidogenic sequences and are able to form amyloid-like substances.
In other words, these spike proteins are similar to those beta-amyloid or tau or alpha-synuclein like substances which may cause neuronal loss in Alzheimer’s or Parkinson’s disease patients.
Their structure makes it easy to form tighter string-like bonded structures with longitudinal twisting as well as cross binding, forming a fibrous-like structure visible under the microscope.
Researchers have found that plasma samples from long COVID patients still contain large anomalous (amyloid) deposits (microclots), which are resistant to fibrinolysis (compared to plasma from controls and diabetes), even after trypsinization (cell dissociation after an enzyme breaks down proteins).
After a second trypsinization, the persistent pellet deposits (microclots) were solubilized. Various inflammatory molecules substantially increased in both the supernatant and trapped in the solubilized pellet deposits of COVID-19, versus that of the control samples.
Of particular interest was a substantial increase in α(2)-antiplasmin (α2AP), various fibrinogen chains, as well as Serum Amyloid A (SAA) that were trapped in the solubilized fibrinolytic-resistant pellet deposits.
Significant abnormal amyloid microclot formation that are resistant to fibrinolysis, increased α2AP, and the surge of acute phase inflammatory molecules may therefore be central contributors in both COVID-19 infection and as well as COVID vaccine-related syndrome.
Spike Protein Inhibits Another Anti-Clot Mechanism
Spike protein is just one surprise after another.
It’s been reported that the spike protein can competitively inhibit the bindings of antithrombin and heparin cofactor II to heparin, causing abnormal increase in thrombin (clotting) activity.
SARS-CoV-2 spike proteins at a similar concentration (~10 μg/mL) as the viral load in critically ill patients can directly cause blood coagulation and thrombosis in the zebrafish model.
To summarize, these unexpected negative effects of spike protein on the dissolving process of blood clots, plus its amyloid nature, all may be the key contributory factors to the abnormal, lengthy fibrous clots observed in COVID-related conditions.
Spike Protein Is the Smoking Gun
There is clinical evidence that the SARS-CoV-2 spike protein has been detected in clots retrieved from COVID-19 patients with acute ischemic stroke and myocardial infarction.
Recent research conducted by cardiologists from the University of Colorado sheds light on the crucial role of spike protein in the pathology of COVID and COVID vaccine-related injuries.
They analyzed seven COVID-19 patients and six mRNA vaccinated patients with myocardial injury and found nearly identical alterations in gene profiling patterns that would predispose them to clotting state, inflammation, and myocardial dysfunction.
In other words, regardless of whether the myocarditis was caused by the virus or vaccine, the expression of genes responsible for prothrombotic state in response to spike protein, inflammation, and myocardial dysfunction, exhibited similar changes.
Based on gene analysis, COVID-19 and post-mRNA vaccine injury have a common molecular mechanism.
The altered genes pattern includes down-regulation in ACE2, ACE2/ACE ratio, AGTR1, and ITGA5, and up-regulation in ACE and F3 (tissue factor).
What is more alarming and not reported before is that microvascular thrombosis has been found in post-vaccinated patients, indicating that spike protein itself is able to trigger blood clots in susceptible patients.
Tip of the Iceberg
Based on the causal relation between ChAdOx1-S vaccines (the AstraZeneca adenovirus COVID vaccine) and thrombosis with thrombocytopenia syndrome, the product information for ChAdOx1-S has been updated to include thrombosis with thrombocytopenia syndrome as a very rare side effect.
This has been named as vaccine induced immune thrombotic thrombocytopenia (VITT), due to the fact that in almost every patient in these reports, high levels of antibodies to platelet factor 4 (PF4)–polyanion complexes were identified in their body.
These unusual blood clots in combination with thrombocytopenia were reported predominantly in women aged under 60 years. Accordingly, several European countries restricted the use of adenovirus vaccines in younger age groups.
This risk has been recently systematically analyzed in an international network cohort study from five European countries and the United States, confirming pooled 30 percent increased risk of thrombocytopenia after a first dose of the ChAdOx1-S vaccine, as well as a trend towards an increased risk of venous thrombosis with thrombocytopenia syndrome after Ad26.COV2.S (the Janssen COVID vaccine) compared with BNT162b2 (the Pfizer-BioNTech COVID vaccine).
However this may be only the tip of the iceberg. There are many more events that could be attributed to the clotting issues including sudden death, cardiovascular events, cardiac death, stroke, disabilities, thrombotic events, etc.
Blood vessels are in all our organs. The vessel problems could explain a wide range of symptoms from the dysfunction to mild decline of our brain, heart, lung, and extremities.
With additional reporting by Enrico Trigoso.


No comments:
Post a Comment