By Tyler Durden: In yet another damning development for the 'safe and effective' crowd, AstraZeneca has announced the worldwide withdrawal of its Oxford-AstraZeneca vaccine, branded as Vaxzevria, due to a serious side effect. This decision marks the end of the vaccine once hailed as a "triumph for British science" by Boris Johnson, The Telegraph reports.
The pharmaceutical giant voluntarily withdrew its "marketing authorization" in the European Union earlier this week, with similar actions expected soon in the UK and other approving countries. The move, described by the company as driven by "commercial reasons," coincides with the availability of newer vaccines designed to combat emerging variants.
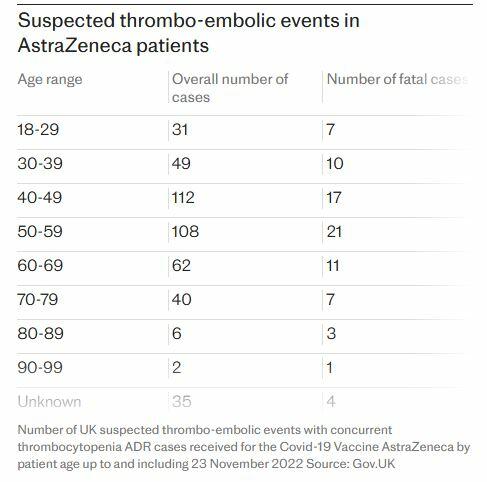
That said, the timing of the withdrawal follows months of intense scrutiny over a rare side effect. In a recent High Court document, the company admitted that Vaxzevria could, in very rare instances, cause Thrombosis with Thrombocytopenia Syndrome (TTS), which has been linked to at least 81 deaths in the UK. Despite these admissions, AstraZeneca maintains that the decision to pull the vaccine is unrelated to the ongoing legal challenges or its serious and deadly side effects.
The European Medicines Agency has begun the process to formally withdraw the vaccine, reflecting an expected move away from monovalent vaccines, which target only the original COVID-19 strain. Marco Cavaleri, head of vaccines at the agency, emphasized that this is a standard procedure for vaccines that are no longer in use.
Legal experts and victims, however, see the withdrawal as a vindication of their long-held concerns over the vaccine's safety. "To those who we represent, all of whom have suffered bereavement or serious injury as a result of the AstraZeneca vaccine, this decision to withdraw marketing authorisation, ending the usage of the AstraZeneca vaccine in the EU, will be welcomed," said Sarah Moore, a partner at Leigh Day, the law firm representing many of the claimants.
"It will be seen as a decision linked with AstraZeneca’s recent admission that the vaccine can cause TTS, and the fact that regulators across the world suspended or stopped usage of the vaccine following concerns regarding TTS."
Victims and their families have reported a range of severe reactions, from fatal thrombosis to lasting disabilities, sparking a debate over the adequacy of vaccine safety monitoring and compensation for vaccine injuries.
Kate Scott, whose husband suffered a permanent brain injury after receiving the vaccine, expressed mixed feelings: "AstraZeneca’s Covid vaccine no longer being used in the UK or Europe, and soon the rest of the world, means no one else will suffer from this awful adverse reaction," she said. "They say it is for commercial reasons, but maybe it’s because it can no longer be seen as being within the acceptable safety parameters, with 445 confirmed cases of VITT, 81 of these fatal in the UK alone."

The government's vaccine damage payment scheme has been criticized for not providing sufficient compensation, prompting calls for reform. "This is an important regulatory step, but still our clients remain without fair compensation. We will continue to fight for the compensation our clients need and campaign for reform of the vaccine damage payment scheme.," Moore added.

No comments:
Post a Comment